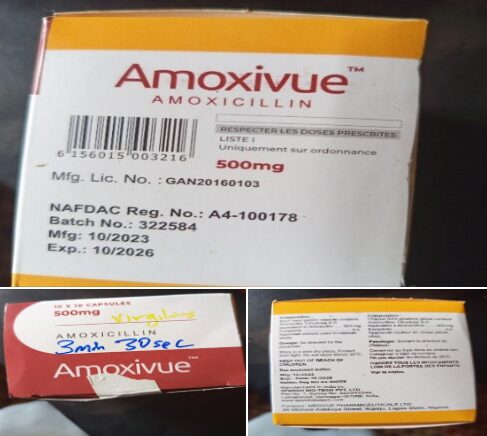
National Agency for Food and Drug Administration and Control (NAFDAC) has announced the recall of Amoxivue (Amoxicillin) 500mg capsules following laboratory tests that revealed dangerously low levels of the active ingredient.
A batch of Amoxivue capsules—Batch No. 322584, manufactured in October 2023 and set to expire in October 2026—was sampled from facilities in Sokoto and two Local Government Areas in Plateau State.
Upon analysis using High-Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared Spectroscopy (FTIR), the capsules were found to contain only 26.3% of the required Active Pharmaceutical Ingredient (API).
Further tests showed that the weight variation and infrared absorption spectrum of the capsules did not meet established pharmaceutical standards, confirming the product as substandard.
Amoxivue (Amoxicillin) 500mg is an antibiotic commonly used to treat respiratory tract infections, ear and sinus infections, urinary tract infections, and skin and soft tissue infections. It works by inhibiting bacterial growth, but insufficient API levels can render it ineffective.
NAFDAC warns that using this substandard antibiotic may lead to treatment failure, development of antibiotic resistance, increased risk of complications, misleading clinical outcomes, and broader public health threats.
The agency urges healthcare professionals and distributors to immediately stop the sale and distribution of the affected batch. Consumers are advised to avoid using the product and report any adverse reactions or suspicions of substandard medicines.
Reports can be made via phone at 0800-162-3322 or by email to [email protected]. NAFDAC has directed its zonal and state offices to intensify surveillance and remove the affected product from circulation
![]()